Processing Your Payment
Please do not leave this page until complete. This can take a few moments.
-
News
-
Editions
-
- Lists
-
Viewpoints
-
HBJ Events
-
Event Info
- 2024 Economic Outlook Webinar Presented by: NBT Bank
- Best Places to Work in Connecticut 2024
- Top 25 Women In Business Awards 2024
- Connecticut's Family Business Awards 2024
- What's Your Story? A Small Business Giveaway 2024 Presented By: Torrington Savings Bank
- 40 Under Forty Awards 2024
- C-Suite and Lifetime Achievement Awards 2024
- Connecticut's Health Care Heroes Awards 2024
-
-
Business Calendar
-
Custom Content
- News
-
Editions
View Digital Editions
Biweekly Issues
- April 15, 2024
- April 1, 2024
- March 18, 2024
- March 4, 2024
- February 19, 2024
- February 5, 2024
- January 22, 2024
- January 8, 2024
- Dec. 11, 2023
- + More
Special Editions
- Lists
- Viewpoints
-
HBJ Events
Event Info
- View all Events
- 2024 Economic Outlook Webinar Presented by: NBT Bank
- Best Places to Work in Connecticut 2024
- Top 25 Women In Business Awards 2024
- Connecticut's Family Business Awards 2024
- What's Your Story? A Small Business Giveaway 2024 Presented By: Torrington Savings Bank
- 40 Under Forty Awards 2024
- C-Suite and Lifetime Achievement Awards 2024
- Connecticut's Health Care Heroes Awards 2024
Award Honorees
- Business Calendar
- Custom Content
Alexion tests drug for possible COVID-19 treatment
 PHOTO | Contributed
Alexion, AstraZeneca Rare Disease's research facility at 100 College St. in New Haven.
PHOTO | Contributed
Alexion, AstraZeneca Rare Disease's research facility at 100 College St. in New Haven.
New Haven-born biotech Alexion Pharmaceuticals has joined the growing list of pharma companies testing their existing drugs to see if they can double as a coronavirus treatment.
The Boston-based company, which has a large research presence in the Elm City, said it will launch a Phase 3 clinical trial next month to see if its rare-disease drug Ultomiris can ease the severe immune response that can lead to potentially fatal lung injury in COVID-19.
The global trial will enroll around 270 patients who have COVID-19 and severe pneumonia or acute respiratory distress syndrome, Alexion said Monday. It will study the drug’s impact on survival, duration of mechanical ventilation and hospital stay.
The FDA approved Ultomiris in December 2018 to treat the rare blood disease, paroxysmal nocturnal hemoglobinuria (PNH). The drug works by inhibiting C5, an immune protein.
Data from animal studies suggest C5 inhibitors could lower levels of cytokine and chemokine, potentially easing the lung inflammation that’s been linked to the deadliest cases of COVID-19, Alexion said.
The company’s original C5 inhibitor, Soliris, has already been given to around 100 COVID-19 patients by independent investigators through “compassionate use.”
Promising early results convinced Alexion that a controlled trial was warranted, said John Orloff, MD, the company’s head of research and development.
Alexion said it decided to test Ultomiris instead of Soliris because it is dosed on a less frequent schedule, reducing the burden for hospitals and allowing it to be manufactured at a higher capacity.
The company has already taken steps to ramp up its supply of the drug, seemingly to avoid the kinds of shortages that lupus and arthritis sufferers have faced with hydroxychloroquine, a drug President Donald Trump has touted as a potential ‘game-changer’ for COVID-19, although it is still being tested.
“As we move quickly to initiate this program, we also remain committed to serving the patients who currently rely on our medicines and providing continuous supply to these patients,” said Orloff in a statement.
* * *
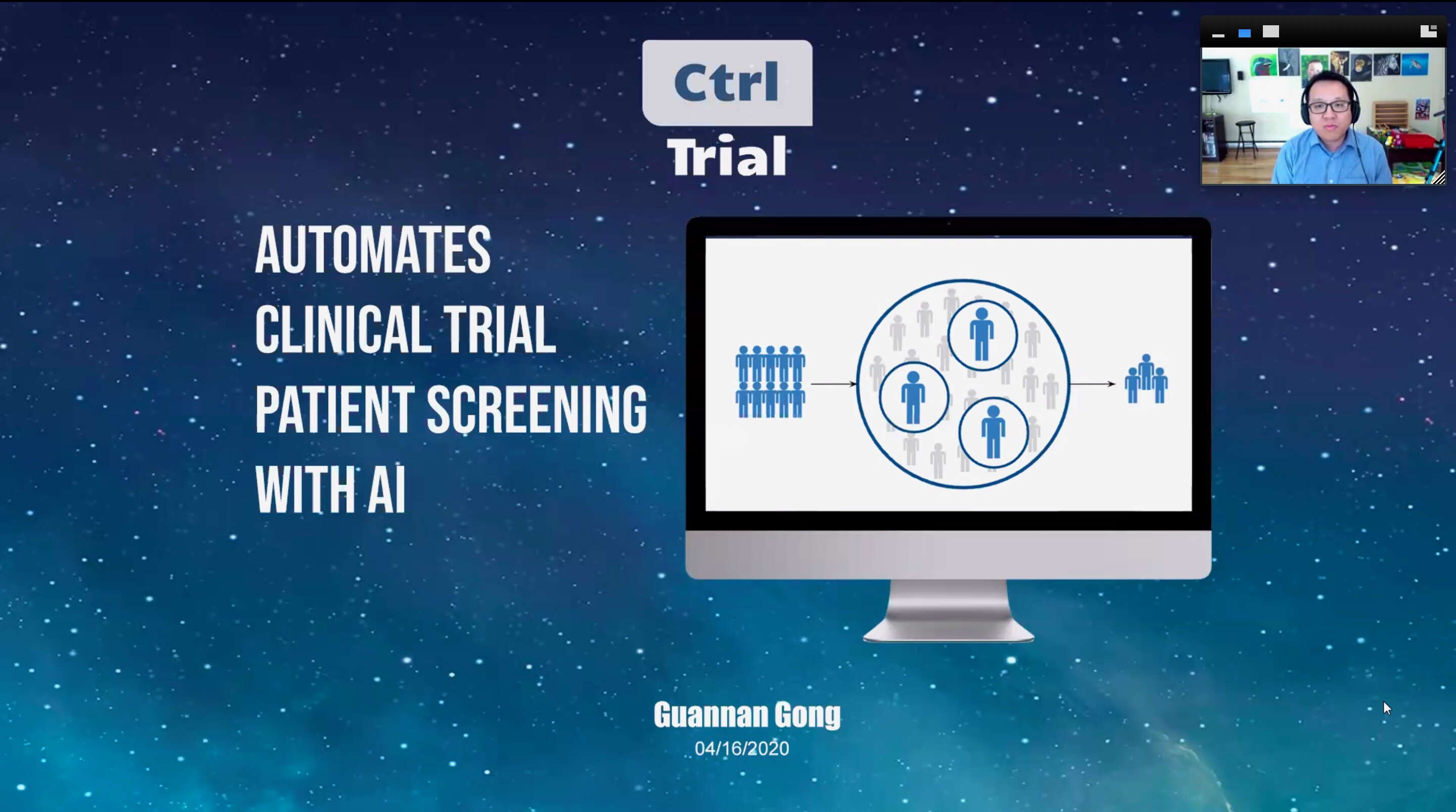
A student-led startup that uses artificial intelligence to match patients with clinical trials of experimental drugs is the 2020 winner of the $15,000 Rothberg Catalyzer Prize at Yale.
The winning venture, Ctrl Trial, aims to speed the patient screening process for recruitment in clinical trials through “automated scanning of clinical trial protocols, patient electronic medical records, and genomics and real-world data.”

The Rothberg Prize, now in its third year, is given to the best student-led venture or project that develops an innovative hardware and/or AI solution to a medical challenge.
It is funded by renowned genomics pioneer and bioscience entrepreneur Jonthan Rothberg. The Yale alumnus and Guilford resident leads the 4Catalyzer bioscience incubator in Guilford and is best known for inventing next-generation high-speed DNA sequencing.
Led by Yale graduate students Guannan Gong, Weiyu Wang and Feimei Lu, Ctrl Trial bested four other student-led teams in a virtual pitch-off event held last Thursday.
The prize was one of four awarded through the annual Startup Yale entrepreneurial award contest. Because of the coronavirus shutdown, Gong and leaders of the other teams gave their pitches from home via Zoom.
Thermaband, a startup that has developed wearable technology to help regulate body temperature, took the audience choice award. Participants in the Zoom conference cast their votes online.
Other contenders were KovaDx, which invented a diagnostic device to rapidly and affordably detect blood disorders, and DM Health, a searchable SaaS platform that helps patients find health-care providers, share health records, maintain treatment plans and compare prices.
The prize is managed by the Yale Center for Biomedical Innovation and Technology and the Tsai Center for Innovative Thinking.
* * *
Biohaven Pharmaceuticals has inked a deal with an Israel-based pharmaceutical firm to distribute its new migraine drug Nurtec ODT in that country.
Biohaven announced the agreement with Medison Pharma last Thursday. Financial terms were not disclosed.
Medison has “strong distribution, medical and access capabilities with a proven track record of delivering innovative products successfully to patients in Israel,” said Donnie McGrath, Biohaven’s head of corporate strategy and business development, in a statement.
Nurtec ODT is a CGRP-antagonist that dissolves in the mouth without water. The U.S. Food & Drug Administration approved the drug for the acute treatment of migraine in February.
Contact Natalie Missakian at news@newhavenbiz.com

2022 Giving Guide
This special edition informs and connects businesses with nonprofit organizations that are aligned with what they care about. Each nonprofit profile provides a crisp snapshot of the organization’s mission, goals, area of service, giving and volunteer opportunities and board leadership.
Learn more
Subscribe
Hartford Business Journal provides the top coverage of news, trends, data, politics and personalities of the area’s business community. Get the news and information you need from the award-winning writers at HBJ. Don’t miss out - subscribe today.
Subscribe
2024 Book of Lists
Delivering Vital Marketplace Content and Context to Senior Decision Makers Throughout Greater Hartford and the State ... All Year Long!
Read Here-
2022 Giving Guide
This special edition informs and connects businesses with nonprofit organizations that are aligned with what they care about. Each nonprofit profile provides a crisp snapshot of the organization’s mission, goals, area of service, giving and volunteer opportunities and board leadership.
-
Subscribe
Hartford Business Journal provides the top coverage of news, trends, data, politics and personalities of the area’s business community. Get the news and information you need from the award-winning writers at HBJ. Don’t miss out - subscribe today.
-
2024 Book of Lists
Delivering Vital Marketplace Content and Context to Senior Decision Makers Throughout Greater Hartford and the State ... All Year Long!
ABOUT
ADVERTISE
NEW ENGLAND BUSINESS MEDIA SITES
No articles left
Get access now
In order to use this feature, we need some information from you. You can also login or register for a free account.
By clicking submit you are agreeing to our cookie usage and Privacy Policy
Already have an account? Login
Already have an account? Login
Want to create an account? Register
Get access now
In order to use this feature, we need some information from you. You can also login or register for a free account.
By clicking submit you are agreeing to our cookie usage and Privacy Policy
Already have an account? Login
Already have an account? Login
Want to create an account? Register






0 Comments