Processing Your Payment
Please do not leave this page until complete. This can take a few moments.
- News
-
Editions
View Digital Editions
Biweekly Issues
- May 13, 2024
- April 29, 2024
- April 15, 2024
- April 1, 2024
- March 18, 2024
- March 4, 2024
- February 19, 2024
- February 5, 2024
- January 22, 2024
- + More
Special Editions
- Lists
- Viewpoints
- HBJ Events
- Business Calendar
- Custom Content
Rothberg’s Hyperfine gets FDA OK to sell portable MRI
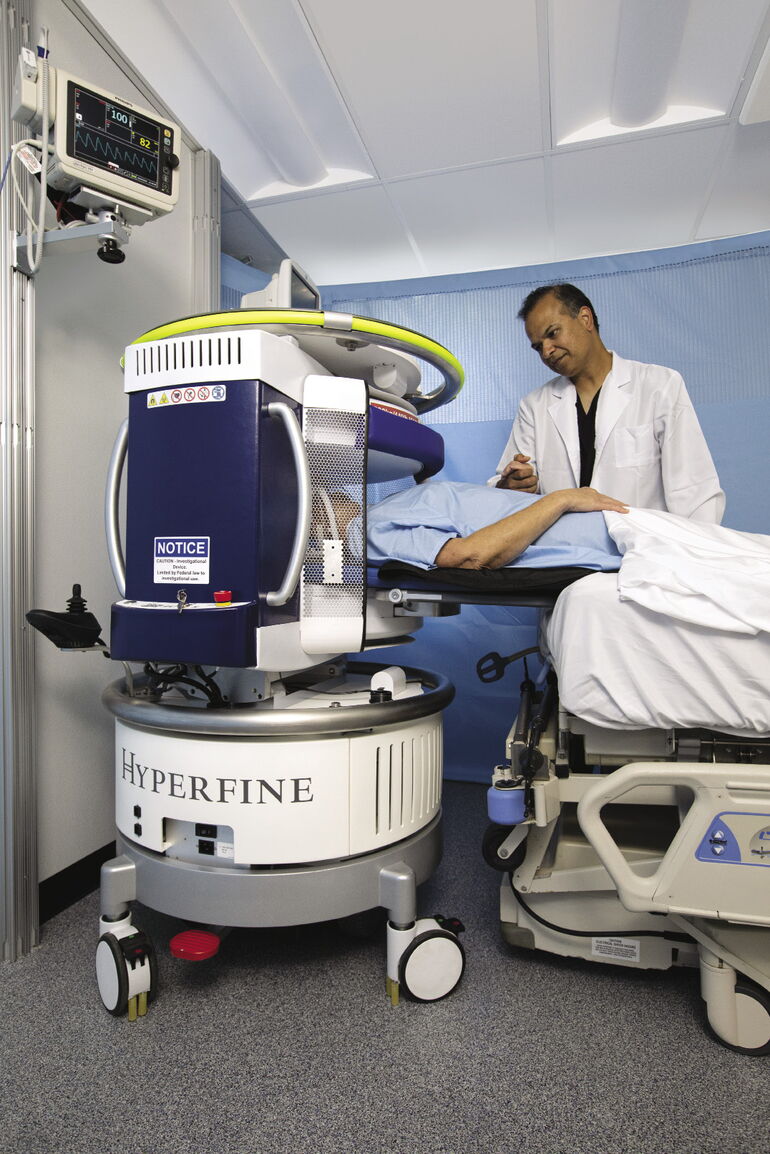 Hyperfine’s portable MRI can be rolled up to a patient’s bedside and produce a 3-D color image of a human brain within 10 minutes.
Hyperfine’s portable MRI can be rolled up to a patient’s bedside and produce a 3-D color image of a human brain within 10 minutes.
A Guilford startup founded by nationally known Connecticut serial entrepreneur Jonathan Rothberg will become the first company to sell a bedside MRI machine after getting the green light from federal regulators.
Hyperfine Research Inc. announced Wednesday morning that it has received 510(k) clearance from the U.S. Food & Drug Administration. The company said it will begin shipping the portable MRI this summer.
Unlike traditional MRI machines that are confined to protected rooms, Hyperfine’s rolls up to the patient’s bedside, plugs into a standard AC wall outlet and is operated with an iPad.
The device is a first in the medical-imaging industry, according to the trade publication Diagnostic Imaging. It is 20 times less expensive, 10 times lighter, and uses 35 times less power than a conventional MRI, according to Hyperfine.
Rothberg has said the company would sell the device for around $50,000, a fraction of the more than $1 million price tag for traditional MRI machines.
“Nearly six years ago, a dream to create a portable, affordable MRI system was born,” Rothberg said in a statement Wednesday. “We assembled a team, and they took the 10 million-fold improvement in computing power since MRI was invented, the best of the billions invested in green electronics and they built something astonishing, something disruptive.”
Rothberg envisions the invention being used not only in hospital rooms, but on cruise ships, in ambulances and at sporting events. He also aims to bring MRI imaging to developing countries. Hyperfine Chief Medical Officer Kahn Siddiqui, MD, said 90 percent of the world has no access to MRI.
“More than 40 years after its first use, MRI remains a marvel. Unfortunately, it also remains inaccessible,” said Siddiqui in a statement. “It’s time that MRI made the jump to point-of-need, just like X-ray and ultrasound have before it.”
“With the FDA’s decision, we are now ready to rewrite the rules of MRI accessibility,” he added.
In testing the device, Hyperfine said thousands of brain scans were performed on patients at Yale New Haven Hospital, Penn Medicine, Good Samaritan Hospital on Long Island, New York Presbyterian Brooklyn Methodist Hospital and Brown University. The FDA clearance is for head imaging of patients age two and older.
Founded in 2014, Hyperfine is based in Rothberg’s 4Catalyzer health technology incubator, which has its flagship office in Guilford. Other locations include New York, Palo Alto, Calif. and Taiwan.
Rothberg, who lives in Guilford, won the National Medal of Technology & Innovation in 2015 for inventing inexpensive and accessible high-speed DNA sequencing. He also founded Guilford startup Butterfly Network, which developed a pocket-sized ultrasound device and has a valuation of more than $1 billion.
Contact Natalie Missakian at news@newhavenbiz.com

2022 Giving Guide
This special edition informs and connects businesses with nonprofit organizations that are aligned with what they care about. Each nonprofit profile provides a crisp snapshot of the organization’s mission, goals, area of service, giving and volunteer opportunities and board leadership.
Learn more
Subscribe
Hartford Business Journal provides the top coverage of news, trends, data, politics and personalities of the area’s business community. Get the news and information you need from the award-winning writers at HBJ. Don’t miss out - subscribe today.
Subscribe
2024 Book of Lists
Delivering Vital Marketplace Content and Context to Senior Decision Makers Throughout Greater Hartford and the State ... All Year Long!
Read Here-
2022 Giving Guide
This special edition informs and connects businesses with nonprofit organizations that are aligned with what they care about. Each nonprofit profile provides a crisp snapshot of the organization’s mission, goals, area of service, giving and volunteer opportunities and board leadership.
-
Subscribe
Hartford Business Journal provides the top coverage of news, trends, data, politics and personalities of the area’s business community. Get the news and information you need from the award-winning writers at HBJ. Don’t miss out - subscribe today.
-
2024 Book of Lists
Delivering Vital Marketplace Content and Context to Senior Decision Makers Throughout Greater Hartford and the State ... All Year Long!
ABOUT
ADVERTISE
NEW ENGLAND BUSINESS MEDIA SITES
No articles left
Get access now
In order to use this feature, we need some information from you. You can also login or register for a free account.
By clicking submit you are agreeing to our cookie usage and Privacy Policy
Already have an account? Login
Already have an account? Login
Want to create an account? Register
Get access now
In order to use this feature, we need some information from you. You can also login or register for a free account.
By clicking submit you are agreeing to our cookie usage and Privacy Policy
Already have an account? Login
Already have an account? Login
Want to create an account? Register






0 Comments