Processing Your Payment
Please do not leave this page until complete. This can take a few moments.
- News
-
Editions
View Digital Editions
Biweekly Issues
- May 13, 2024
- April 29, 2024
- April 15, 2024
- April 1, 2024
- March 18, 2024
- March 4, 2024
- February 19, 2024
- February 5, 2024
- January 22, 2024
- + More
Special Editions
- Lists
- Viewpoints
- HBJ Events
- Business Calendar
- Custom Content
FDA clears AI software for Hyperfine’s portable MRI
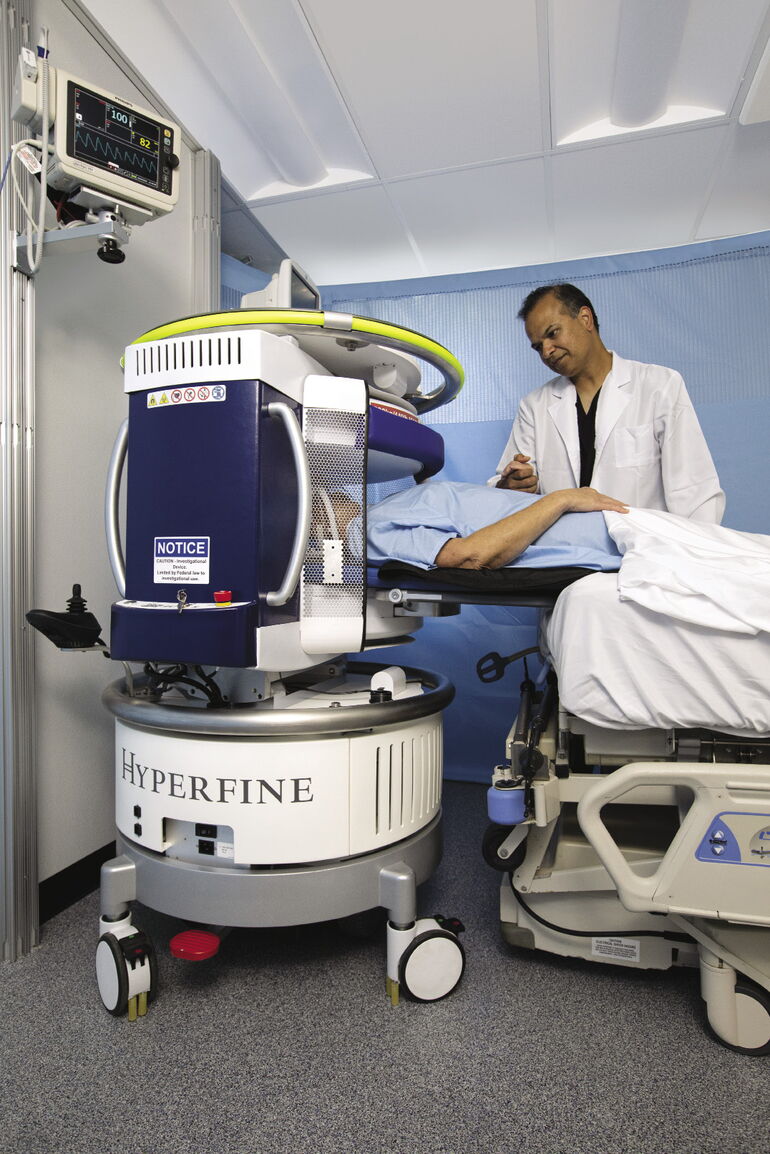 Hyperfine’s portable MRI can be rolled up to a patient’s bedside and produce a 3-D color image of a human brain within 10 minutes.
Hyperfine’s portable MRI can be rolled up to a patient’s bedside and produce a 3-D color image of a human brain within 10 minutes.
Federal regulators have given the green light to artificial intelligence software for a new portable MRI machine being marketed by a Guilford company.
The Food and Drug Administration granted 510(k) clearance to Hyperfine’s Advanced AI image software for the company’s portable MRI machine, Swoop.
Company officials say the AI software will help doctors analyze scans and diagnose brain diseases, such as strokes. The Advanced AI Applications analyze and return annotated and segmented brain images within minutes of scanning.
“With this powerful tool now built into the Swoop system, we are making MR imaging not only accessible at the bedside, but making it easier for providers to move quickly from scan to a recommended course of treatment,” said Hyperfine Chief Medical Officer Dr. Khan Siddiqui in a statement.
While traditional MRI machines must be confined to protected rooms, Hyperfine’s rolls up to the patient’s bedside, plugs into a standard AC wall outlet and is operated with an iPad.
Hyperfine, founded by prominent Connecticut bioscience entrepreneur, genomics pioneer and New Haven native Jonathan Rothberg, began marketing the device this summer at a fraction of the cost of conventional machines.
The FDA cleared the machine last year to take brain images in patients ranging from newborns to adults.
Read a previous New Haven Biz story on Hyperfine’s portable MRI here.
Contact Natalie Missakian at news@newhavenbiz.com

2022 Giving Guide
This special edition informs and connects businesses with nonprofit organizations that are aligned with what they care about. Each nonprofit profile provides a crisp snapshot of the organization’s mission, goals, area of service, giving and volunteer opportunities and board leadership.
Learn more
Subscribe
Hartford Business Journal provides the top coverage of news, trends, data, politics and personalities of the area’s business community. Get the news and information you need from the award-winning writers at HBJ. Don’t miss out - subscribe today.
Subscribe
2024 Book of Lists
Delivering Vital Marketplace Content and Context to Senior Decision Makers Throughout Greater Hartford and the State ... All Year Long!
Read Here-
2022 Giving Guide
This special edition informs and connects businesses with nonprofit organizations that are aligned with what they care about. Each nonprofit profile provides a crisp snapshot of the organization’s mission, goals, area of service, giving and volunteer opportunities and board leadership.
-
Subscribe
Hartford Business Journal provides the top coverage of news, trends, data, politics and personalities of the area’s business community. Get the news and information you need from the award-winning writers at HBJ. Don’t miss out - subscribe today.
-
2024 Book of Lists
Delivering Vital Marketplace Content and Context to Senior Decision Makers Throughout Greater Hartford and the State ... All Year Long!
ABOUT
ADVERTISE
NEW ENGLAND BUSINESS MEDIA SITES
No articles left
Get access now
In order to use this feature, we need some information from you. You can also login or register for a free account.
By clicking submit you are agreeing to our cookie usage and Privacy Policy
Already have an account? Login
Already have an account? Login
Want to create an account? Register
Get access now
In order to use this feature, we need some information from you. You can also login or register for a free account.
By clicking submit you are agreeing to our cookie usage and Privacy Policy
Already have an account? Login
Already have an account? Login
Want to create an account? Register






0 Comments